One Portal Researcher Tools
The Clinical Trial Center (CTC) strives to provide the clinical researcher with all the tools necessary to make your clinical study a straightforward, streamlined process. Clinical researchers at Loma Linda University Health (LLUH) are invited to take full advantage of resources available through our One Portal Intranet site. Access is only available while on campus and only with LLUH computer stations.
Use the Online Submission Form to submit a new study, a study amendment, or study closure.
Manage Your Clinical Study (Access via One Portal Intranet)
Processing a clinical study from onset to completion is a complex project. Careful planning is crucial in order to avoid any delays. Understanding the design and process of clinical studies can provide investigators and their staff with a comprehensive outline and detailed steps of the required processes in order to achieve the successful and efficient conduct of clinical studies.Information about processes for clinical studies and how they are designed is available on the Clinical Trials One Portal Intranet site. One Portal access is required.
Primary Investigators and Department Administrators can view the financial status of their Clinical Trials using the Clinical Trial Center Dashboard.
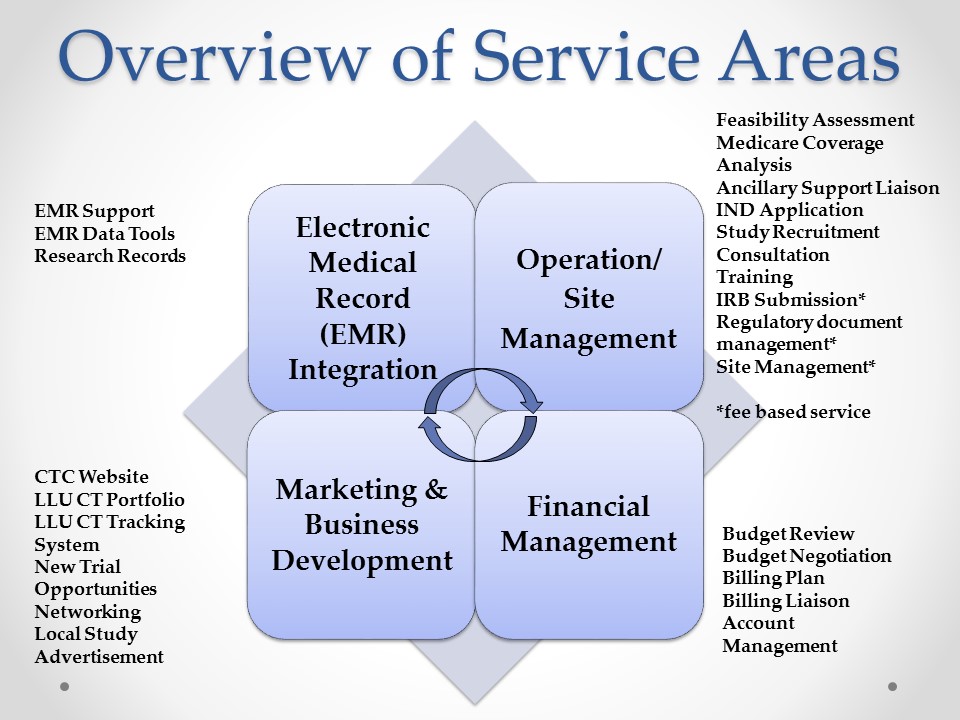
Clinical Trial Center Services
The Clinical Trial Center is proud to offer a variety of services as highlighted below. We seek to partner with the Primary Investigator and research teams to support your clinical research activity. We will alsoserve as your primary contact during the study start up process. This leaves time for the principal investigator and study staff to plan for the start-up of the trial. Please reach out to the CTC if you have any questions.
Clinical Trial Center Study Process
This chart provides an at a glance look at the life cycle and processes involved with clinical research. CTC support is available every step of the way and serves as your primary contact with the study sponsor during your start-up process, which includes study budget and billing items. In addition, the CTC serves as the primary contact for budget amendment or payment concerns throughout the life of your study. Please feel free to contact the CTC with any questions or for assistance throughout the life cycle.